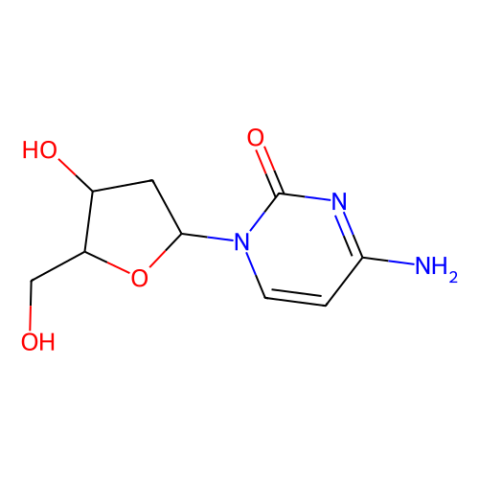
2’-脱氧胞苷
- CAS编号: 951-77-9
- 分子式: C9H13N3O4
- 分子量: 227.22
- Beilstein号: 87567
- EC号: 213-454-1
- MDL号: MFCD00006547
- PubChem编号: 13711
库存信息
库存信息
库存信息
库存信息
库存信息
| 货号 (SKU) | 包装规格 | 是否现货 | 价格 | 数量 |
|---|---|---|---|---|
| D104773-500mg |
500mg |
现货  |
| |
| D104773-1g |
1g |
现货  |
| |
| D104773-5g |
5g |
现货  |
| |
| D104773-25g |
25g |
现货  |
| |
| D104773-100g |
100g |
现货  |
|
 首页
首页 400-620-6333
400-620-6333



 危险品化学品经营许可证(带存储)
危险品化学品经营许可证(带存储)